
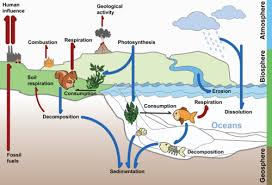
These maps show net primary productivity (the amount of carbon consumed by plants) on land (green) and in the oceans (blue) during August and December, 2010. In the fall and winter, as vegetation dies back in the northern hemisphere, decomposition and respiration returns carbon dioxide to the atmosphere. This cycle peaks in August, with about 2 parts per million of carbon dioxide drawn out of the atmosphere. This graph shows the difference in carbon dioxide levels from the previous month, with the long-term trend removed. As the large land masses of Northern Hemisphere green in the spring and summer, they draw carbon out of the atmosphere. The ebb and flow of the fast carbon cycle is visible in the changing seasons. During the spring, when plants begin growing again, concentrations drop. In the Northern Hemisphere winter, when few land plants are growing and many are decaying, atmospheric carbon dioxide concentrations climb. The fast carbon cycle is so tightly tied to plant life that the growing season can be seen by the way carbon dioxide fluctuates in the atmosphere. In all four processes, the carbon dioxide released in the reaction usually ends up in the atmosphere. The basic chemical reaction looks like this: In each case, oxygen combines with sugar to release water, carbon dioxide, and energy. Plants and plankton die and decay (are eaten by bacteria) at the end of the growing season. Animals (including people) eat the plants or plankton, and break down the plant sugar to get energy. Plants break down the sugar to get the energy they need to grow. The chemical reaction looks like this:įour things can happen to move carbon from a plant and return it to the atmosphere, but all involve the same chemical reaction. Using energy from the Sun, both plants and plankton combine carbon dioxide (CO 2) and water to form sugar (CH 2O) and oxygen.

Phytoplankton (microscopic organisms in the ocean) and plants take carbon dioxide from the atmosphere by absorbing it into their cells. Plants and phytoplankton are the main components of the fast carbon cycle. (See The Ocean’s Carbon Balance on the Earth Observatory.) It is likely that changes in ocean temperatures and currents helped remove carbon from and then restore carbon to the atmosphere over the few thousand years in which the ice ages began and ended. In the meantime, winds, currents, and temperature control the rate at which the ocean takes carbon dioxide from the atmosphere. Over millennia, the ocean will absorb up to 85 percent of the extra carbon people have put into the atmosphere by burning fossil fuels, but the process is slow because it is tied to the movement of water from the ocean’s surface to its depths. However, since carbon concentrations in the atmosphere have increased, the ocean now takes more carbon from the atmosphere than it releases. The hydrogen reacts with carbonate from rock weathering to produce bicarbonate ions.īefore the industrial age, the ocean vented carbon dioxide to the atmosphere in balance with the carbon the ocean received during rock weathering. Once in the ocean, carbon dioxide gas reacts with water molecules to release hydrogen, making the ocean more acidic. At the surface, where air meets water, carbon dioxide gas dissolves in and ventilates out of the ocean in a steady exchange with the atmosphere. However, the slow carbon cycle also contains a slightly faster component: the ocean. It takes a few hundred thousand years to rebalance the slow carbon cycle through chemical weathering. If carbon dioxide rises in the atmosphere because of an increase in volcanic activity, for example, temperatures rise, leading to more rain, which dissolves more rock, creating more ions that will eventually deposit more carbon on the ocean floor. For comparison, humans emit about 30 billion tons of carbon dioxide per year-100–300 times more than volcanoes-by burning fossil fuels.Ĭhemistry regulates this dance between ocean, land, and atmosphere. At present, volcanoes emit between 130 and 380 million metric tons of carbon dioxide per year. When volcanoes erupt, they vent the gas to the atmosphere and cover the land with fresh silicate rock to begin the cycle again. The heated rock recombines into silicate minerals, releasing carbon dioxide. When the plates collide, one sinks beneath the other, and the rock it carries melts under the extreme heat and pressure. Earth’s land and ocean surfaces sit on several moving crustal plates. The slow cycle returns carbon to the atmosphere through volcanoes.


 0 kommentar(er)
0 kommentar(er)
